Why fertilize?
Fertilizer provides a source of essential nutrients that plants need to grow normally and be healthy. Nutrients are also supplied by the soil and decomposing organic matter, but adequate quantities of certain nutrients may be lacking. Young shrubs and trees in landscape beds often benefit from fertilization. Fertilizers can stimulate growth and improve the color and appearance of plants. The three primary nutrients contained in most fertilizers are nitrogen, phosphorus and potassium.
Nitrogen is the fertilizer nutrient that plants use in the largest amount, and fertilizing with nitrogen causes plants to green up and increase growth rate. Established plants that are putting on very little new growth each year or whose leaves are small and light in color may be showing signs of nitrogen deficiency. Unfortunately, there is no lab test for nitrogen because it has many forms and can be converted or lost quickly in the soil environment. Nitrogen recommendations are based on research and experience with how plants respond to added nitrogen.
It is unlikely that trees or shrubs will respond to phosphorus fertilization unless soil levels are very low. Many NH soils are already high in phosphorus and more should not be added unless recommended by a soil test. Excess phosphorus in the environment may be carried by stormwater runoff into nearby surface waters, where it can contribute to water quality degradation.
Potassium is generally low in our soils, so can be included in routine annual fertilization along with nitrogen. The amount should be determined by a soil test. Areas of dead tissue along leaf edges may be a symptom of potassium deficiency (but may be caused by drought or other factors).
Besides nitrogen, phosphorus and potassium, minor nutrients such as iron, magnesium or manganese are sometimes deficient in landscape plants. These deficiencies can be diagnosed by plant symptoms, soil tests and/or leaf tissue analyses. Problems caused by planting too deep, drought, over-watering, disease or mechanical damage may cause symptoms similar to nutrient deficiencies. Fertilizer applications will not correct cultural problems.
A soil test is useful in determining and adjusting pH, which is important for the availability of soil nutrients. A standard soil test includes pH, phosphorus, potassium, calcium and magnesium. Minor nutrients (micronutrients) can be requested as an additional test. Visit our Soil Testing Services page for soil test forms and instructions.
Many trees and shrubs in the home landscape already have an adequate supply of nutrients available from the soil, decomposing organic matter, residual additions of nutrients, and/or from lawn fertilizers routinely applied around them. As long as a tree or shrub appears healthy and is growing at an acceptable rate, there is no need to give it any additional fertilizer. Mature trees in a natural environment seldom require fertilizer due to their extensive root systems and symbiotic relationships with naturally occurring soil micro-organisms.
Did You Know?
As long as a tree or shrub appears healthy and is growing at an acceptable rate, there is no need to give it any additional fertilizer.
Many NH soils are already high in phosphorus and more should not be added unless recommended by a soil test.

When to apply fertilizer
Newly planted trees and shrubs lack the ability to absorb nutrients until they grow an adequate root system. Fertilizing at planting with quicklyavailable nutrient sources is not recommended and may actually inhibit root growth. However, a soil test is useful to determine the need for adjusting pH before planting. If the soil test indicates a need for phosphorus or potassium, incorporate these nutrients into the bed or backfill at planting time as directed.
For established plants, apply fertilizer in spring before growth starts, or wait until mid-summer after shoot growth ceases, as root growth and maximum nutrient uptake occurs during these periods. Plants generally take in few nutrients during periods of active shoot growth or flowering. Avoid fertilizing with high nitrogen sources during late summer (late August to mid-September), because it may cause certain plants to put out a new flush of leaves that could be susceptible to early frost damage. Fall fertilization (mid-September through mid-October) can be beneficial, however, providing plants with nutrients that are stored in roots and stems, ready to use for the next spring’s growth.
Do not fertilize plants showing symptoms of drought stress. Do not fertilize a tree or shrub during a drought unless plants are irrigated regularly. Plants cannot use the fertilizer without adequate water. Some fertilizers may damage the roots and scorch the leaves if water is lacking.
Do not apply fertilizer indiscriminately. Lawn and landscape fertilizers, if not managed properly, are a contributing source of nutrients associated with water quality degradation. Excess nitrogen can leach through soil and pollute ground water; it is also of high concern in estuarine systems. Phosphorus carried by runoff water into freshwater lakes, ponds, rivers or streams can result in algae blooms and have other biological effects. In addition, the effects of diseases, insects or environmental stresses may be more severe on heavily-fertilized plants.
Managing soil pH
Unaltered NH soils commonly range in pH from 4.5 to 5.5. A standard soil test will determine your soil pH level and recommend the amendments needed to raise or lower pH to provide the optimum environment for trees and shrubs growing there.
Most deciduous trees and shrubs do best within a soil pH range of 5.5 to 6.8. Red maples, oaks, junipers and most conifers (pines, firs and hemlocks) prefer a pH of 5.5 to 6.0. Some conifers can tolerate higher levels; for example, yews and arborvitae prefer a pH of 6.0 to 7.0. Deciduous plants that are tolerant of a higher pH (7.0 or above) include lilacs, pink or white hydrangeas, and some viburnums. Big leaf hydrangea (Hydrangea macrophylla) is unique in its response to soil pH, producing blue flowers in low-pH (acid) soils and pink flowers in high pH (alkaline) soils.
Incorporating 5-10 lbs. of ground limestone per 100 square feet of area will generally raise pH one-half to one unit on the pH scale, but a more precise recommendation can be determined by a soil test. It is best to incorporate lime into the soil to a depth of 6 inches before planting, since surface applications are slow to change pH levels. If applying lime to established plantings, broadcast it on the soil surface and rake it in lightly.
Wood ashes from household stoves will also raise pH; use twice as much wood ash by weight as the recommendation for limestone. Do not over-apply as wood ash is highly soluble and raises pH much more rapidly than limestone. Do not use ashes from coal stoves because coal ash contains elements toxic to plants.
If necessary, pH can be lowered by applying elemental sulfur to the soil. Apply 1- 1 ½ lbs. per 100 square feet and repeat after 3-4 weeks if necessary. Incorporate it before planting if possible. Do not apply more than 1 lb. per application if plants are present, as it may burn the roots.
Did You Know?
Do not fertilize plants showing symptoms of drought stress, and do not fertilize during drought.
What fertilizers to use
Complete fertilizers contain the three major plant nutrients: nitrogen (N), phosphorus (as phosphoric acid, P2O5) and potassium (as potash, K2O). Complete fertilizers are commonly used in home landscapes because they are readily available and reasonably inexpensive. However, applying nutrients that are not needed is wasteful and not recommended because of environmental concerns.
The fertilizer label (Fig. 1) identifies the percentages of nitrogen, phosphorus, and potassium, respectively, as a set of three numbers, e.g., 15-5-10. This is called the fertilizer grade or analysis. They are always listed in the same order. In this example, the fertilizer contains 15 percent N, 5 percent P2O5, and 10 percent K2O by weight. The ratio of nutrients in this fertilizer, obtained by dividing by the lowest common denominator, is 3-1-2. When a complete fertilizer is used on woody plants, the ratio should be approximately 3-1-2 or 3-1-1 if all three nutrients are needed. Examples of fertilizers with these ratios include 24-8-16, 15-5-10, and 15-5-5.

The sources of nutrients are identified on the fertilizer label. Synthetic, soluble sources of nitrogen include materials such as ammonium nitrate, ammonium sulfate and urea. They are low in cost and are rapidly available to the plant, producing a quick growth and green-up response, but they do not last long in the soil environment. Over-application of soluble fertilizers may burn plants.
A better choice for home landscapes is one of the slow-release fertilizers, which may be made up of synthetic or natural organic slow release sources of nutrients. Look carefully at the fertilizer bag or label to see how much slow-release nitrogen it contains. Choose a slow-release fertilizer that contains at least 30 - 50 percent slow-release or “waterinsoluble nitrogen,” if possible. Slow-release fertilizers are more expensive than soluble sources but will provide a consistent supply of nitrogen to the plant throughout the growing season. Chances of overdosing or burning plants with slow-release fertilizer are very low. Slow-release fertilizers are also considered environmentally-friendly since only small amounts of nitrogen are released at a time. Common slow-release synthetic nitrogen sources include sulfur-coated urea (SCU), isobutizidene diurea (IBDU) and plastic- or resin-coated prills.
Organic materials such as compost and manures are natural slowrelease sources of nutrients. Because they contain a low percentage of nitrogen they must be used in relatively large quantities to supply the desired levels of nutrients. Animal manure varies in its nutrient content but typically contains less than one percent of each nutrient. One drawback to using either natural or synthetic slow-release fertilizers is that most of them are complete fertilizers and may result in overapplication of phosphorus.
Recycling organic matter in your landscape is an excellent way to avoid nutrient deficiencies and keep yard waste on site. It is estimated that leaves which fall from a tree and decompose in place recycle at least a pound of nitrogen per 1000 square feet of surface area. You can allow several inches of leaves to naturally accumulate in the fall (and serve as mulch) in shrub beds and tree areas, if there are not ground cover plants underneath. If you prefer to rake up leaves and trimmings, shred and compost them and reapply the finished compost to the planted areas.
A number of special fertilizers on the market have been developed for certain uses, such as rose fertilizer or azalea fertilizer. Use the same criteria to evaluate these fertilizers as discussed above. Acid-forming fertilizers are useful for maintaining low soil pH for acid-loving plants such as azaleas, rhododendrons, camellias, mountain laurel, pieris, hollies and blueberries. However, long term use of these fertilizers may actually make the soil too acidic, so check the pH at least once every 2-3 years.
How much to apply
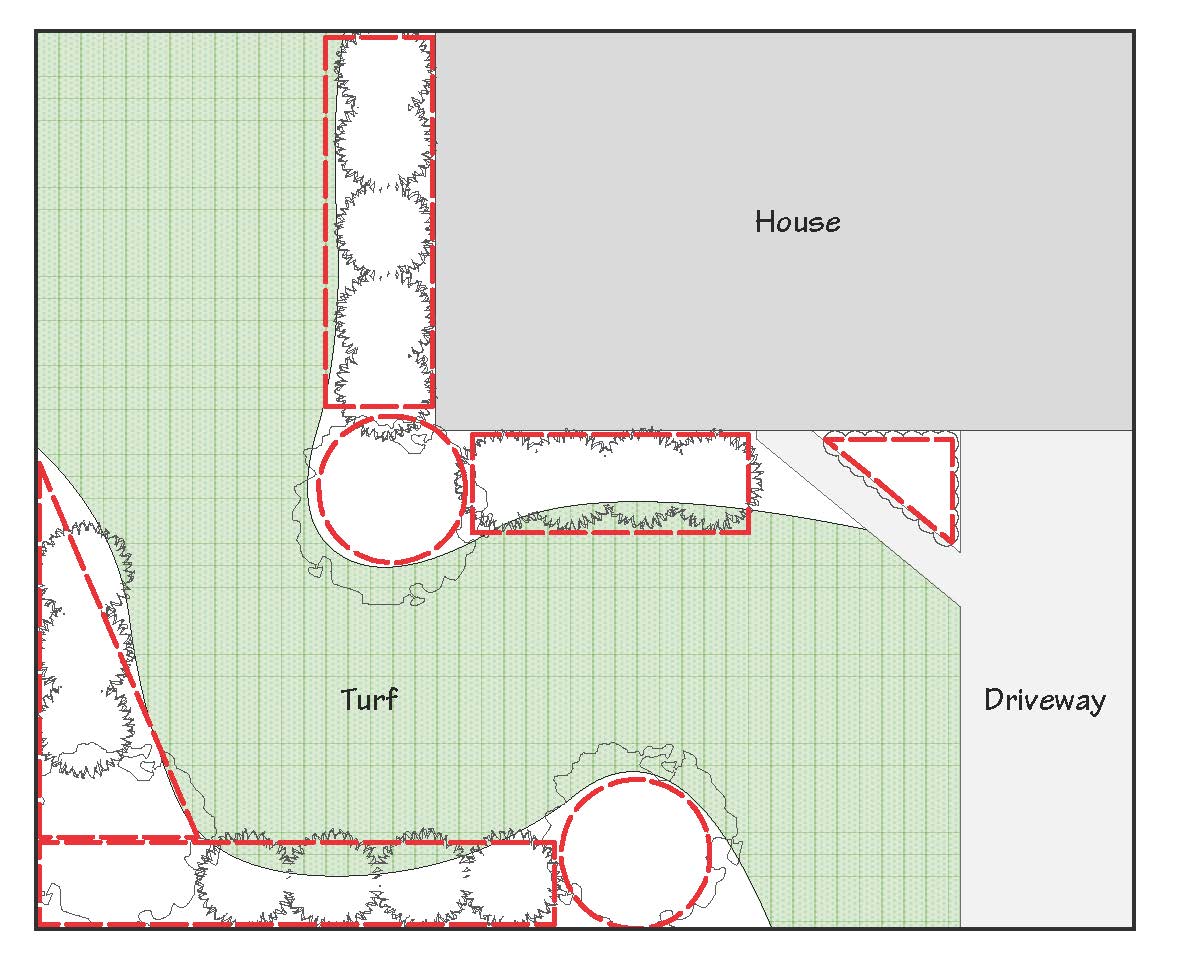
The amount of fertilizer to apply is based on the area of the plant bed or calculated root zone of the plants or trees. For landscape beds, roughly calculate the area to be fertilized by using these formulas:
• square or rectangular areas: length in feet x width in feet = area in square feet
• circular areas: the radius of the circle (in feet) times itself, times 3.14 = area in square feet
• half circular areas: ½ times the radius (in feet) times itself, x 3.14 = area in square feet
• triangular areas: ½ times the length in feet x maximum width in feet = area in square feet
Most landscape beds can be visualized as some combination of these geometric shapes Figure 2). Add them together to get the total square feet of area to be fertilized.
For individual trees, fertilize an area up to one and one half times the canopy diameter. (A tree’s canopy is the area within the tips of its branches.) If the tree is bordered by a recently fertilized lawn, to avoid overfeeding the lawn, do not apply fertilizer to the grass; instead, measure and fertilize only the mulched or bare area under the tree. After several years of growth in the landscape, trees will not need fertilizer unless symptoms of nutrient deficiencies develop.
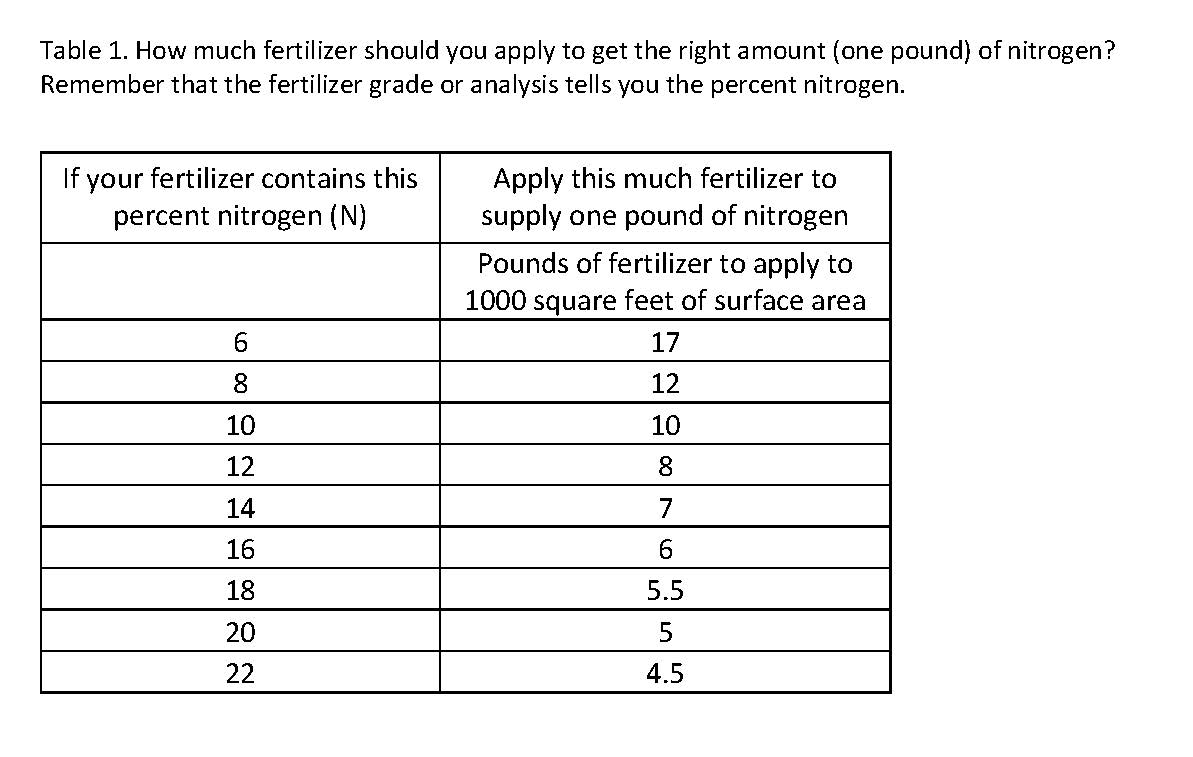
Apply one to three pounds of actual nitrogen (see explanation below) per 1000 square feet of surface area to be fertilized. Rhododendrons, azaleas and other plants with very shallow root written by Catherine A. Neal, UNH Cooperative Extension Ornamentals Specialist, 5/00 systems should be fertilized at the low rate. Plants that tend to put on excessive growth such as forsythia, honeysuckle, privet, willow, Siberian elm and silver maple require little or no nitrogen fertilization. To promote moderate growth of other established trees and shrubs use two pounds. Use rates higher than two pounds, from slow-release nitrogen sources only, when rapid growth rates are desirable. To calculate the amount of a particular fertilizer that will provide one pound of actual nitrogen, divide the percent nitrogen listed on the bag into 100. For example, a 15-5-10 fertilizer contains 15% nitrogen. Fifteen into 100 equals 6.6, therefore, apply 6.6 (rounded off to 6.5) pounds of this fertilizer per 1000 square feet. Twice as much, or 13 pounds, would be required to provide two pounds of nitrogen per 1000 square feet.
How to apply fertilizer
The simplest and fastest way to fertilize trees and shrubs is to broadcast granular fertilizer evenly over the bed or root zone. Subsurface applications of dry or liquid fertilizers are no more effective than broadcast methods in most circumstances, but can help prevent fertilizer runoff from a steep slope. Fertilizer stakes or spikes are expensive and inefficient ways to fertilize. Deep-root feeding is not generally advantageous.
Minor element deficiencies may also be corrected by spraying liquid fertilizer on the leaves, a practice called foliar feeding. This is not an effective practice for nitrogen or phosphorus fertilization, but can be used to provide iron, manganese or other minor nutrients directly to the leaves. A long-term solution to the problem requires correction of underlying site problems, soil pH adjustment and/or soil applications of the deficient nutrients.

About the Author: Dr. Cathy Neal is an Extension Professor and Specialist in nursery and landscape horticulture with University of New Hampshire Cooperative Extension. Her programs emphasize sustainable landscape practices that protect our natural resources. She is also a researcher with the NH Agricultural Experiment Station in Durham, NH, where she conducts field research on meadow establishment and pollinator habitat.
For More Information
State Office
Taylor Hall
59 College Rd.
Durham, NH 03824
http://extension.unh.edu
Education Center and Information Line
answers@unh.edu
1-877-EXT-GROW
(1-877-398-4769)
9 am-2 pm M-F
Search key words:
"UNH Education Center"
Download the resource for the complete fact sheet and a printable version.

