A guide for community officials, planners, and natural resource professionals
A 25-page guide produced in cooperation with the University of New Hampshire Cooperative Extension, the UNH Department of Natural Resources, and the NH Fish and Game Department. This project was supported by funds from the sale of the Conservation License Plate (Moose Plate) under the NH State Conservation Committee grant program.
Abstract
The hydroperiod of a wetland (the length of time and portion of year the wetland holds ponded water) is largely responsible for determining what amphibian species can breed successfully in the wetland. Hydroperiod determines not only the length of time that amphibian larvae have for developing to the point where they can leave the water for land, but also the number and types of predators to which they are exposed. Wetlands can be grouped into three major hydroperiod categories (short, intermediate, and long). Wetlands within each category support a unique collection of amphibian species and together they support the entire diversity of pond-breeding amphibians in New Hampshire. To maintain a diversity of pond-breeding amphibians, we must maintain a diversity of wetlands with different hydroperiods across the landscape. Additionally, upland habitats provide amphibians with wintering and feeding habitat, as well as critical dispersal corridors between wetlands. We must protect uplands if we want to maintain functioning populations of pond-breeding amphibians into the future.
Table of Contents
- Abstract
- Introduction
- Basic life cycle of pond-breeding amphibians
- Wetlands occur along a hydrologic gradient
- How hydroperiod affects amphibian use of wetlands
- Role of amphibian larval period
- Role of aquatic predator
- Amphibian defenses against predators
- Which wetlands support the greatest diversity of amphibian species? .
- Maintaining amphibian diversity
- Maintain wetlands of every hydroperiod
- Identifying wetland hydroperiod
- Suggestions for assessing wetland hydroperiod
- Site indicators for predicting wetland hydroperiod
- When to conduct hydroperiod assessments
- How many wetlands must be protected?
- Conserving wetlands across the landscape
- Wetland size is a poor indicator of importance
- Importance of amphibian dispersal
- Maintain intact uplands around and between wetlands
- Other Resources
- Works Referenced
- About the Authors
Introduction
More than three quarters of New Hampshires amphibian species require ponded wetlands for their breeding habitat. Lakes, ponds, emergent marshes, scrub-shrub swamps, wet meadows, vernal pools, and other forested wetlands provide standing water that many frog and salamander species use for breeding and for depositing their eggs. Each wetland possesses different characteristics that determine its suitability as breeding habitat for amphibians. As a result, not all amphibians are found in all types of wetlands.
An important variable that determines a wetlands suitability as amphibian breeding habitat is its hydroperiod the length of time and portion of the year the wetland holds water. Wetlands vary in their hydroperiod from very short (holding water for less than a few weeks each year) to very long or permanent (lakes and ponds). Between these extremes are wetlands that hold water for various lengths of time during the year, including some wetlands that dry only in years when there is very little precipitation.
A wetlands hydroperiod determines not only the length of time available for amphibian larvae (e.g., tadpoles) to develop into juveniles, but also the ability of the wetland to provide habitat for fish and aquatic insects which are important predators of amphibian larvae and eggs. As such, wetland hydroperiod is largely responsible for determining which amphibian species use different wetlands, and it influences complex predator-prey interactions that determine whether amphibian breeding efforts within a wetland will succeed or fail.
Natural resource professionals and community planners interested in protecting and conserving amphibian diversity will make better management decisions if they understand the important role wetland hydroperiod plays in determining habitat use and distribution of pond-breeding amphibians. Assessing and understanding wetland hydroperiod is an important first step toward guiding management decisions aimed at minimizing or avoiding loss or degradation of individual wetlands that provide significant amphibian breeding habitat in an area.
This guide summarizes the current understanding of wetland hydroperiod and how it influences the distribution of pond-breeding amphibians in New Hampshire. It provides suggestions for identifying and assessing wetlands in New Hampshire based on their hydroperiod. Finally, it provides recommendations for guiding land management practices aimed at maintaining wetland diversity and connectivity, two important factors for maintaining viable amphibian populations throughout the state.
Basic life cycle of pond-breeding amphibians
Six salamander species and 10 frog species breed in ponded wetlands in New Hampshire (Table 1 provides a full list of these species, including their average breeding dates and egg-laying dates). Adults of most of these species spend a portion of their year in upland habitats in the leaf litter and/or under surface objects such as stones and fallen logs or in underground burrows. Some species, such as spotted salamanders, may remain in upland habitats for more than 11 months of the year, while others, such as bullfrogs, spend most of the year in aquatic habitats. All these species, however, require water for breeding and most migrate to wetlands during the breeding season (Table 1).
Three Reproductive Strategies of Pond-Breeding Amphibians
Most pond breeding amphibians, including spotted salamanders and wood frogs, migrate to breeding ponds in spring or summer where they mate and deposit their eggs directly in the water. Eggs hatch into free-swimming aquatic larvae that remain in the pond until they metamorphose into juveniles, complete with legs and lungs, and then they leave the wetland for terrestrial habitats.
Four-toed salamanders migrate to breeding ponds in the spring, but they deposit their eggs in a nest within clumps of moist sphagnum moss growing on and
overhanging hummocks within breeding wetlands. The female guards the eggs until they hatch, at which time the free-swimming larvae wriggle down through the sphagnum moss and drop into the water of the pond where they continue their development.
Marbled salamanders lay their eggs in a nest within the leaf litter of a dry pond in autumn.The female guards the eggs until the pond fills with water in late autumn; the free-swimming larvae hatch, over-winter in the pool, and emerge as juveniles the following spring or early summer.
Table 1. New Hampshires pond-breeding amphibians, their breeding dates, egg and larval periods, and approximate time of
metamorphosis
Salamanders
- Species: Marbled salamander
- Breeding period & egg deposition: September to October
- Time to hatching (days): 9 to 15 but see comments
- Larval period: >100 days
- Metamorphosis: May through June
- Comments: Adults deposit eggs in dry pool bed in But see autumn. Eggs develop and then hatch comments within 1-2 days of the pool filling with water in autumn or spring. Larvae hatched in autumn will overwinter in pool and begin developing in spring. Listed as an endangered species in New Hampshire
- Species: Jefferson salamander
- Breeding period & egg deposition: March to April
- Time to hatching (days): 13 to 45
- Larval period: 56 to 125 days
- Metamorphosis: June through mid-August
- Comments:Vernal pool indicator species in New Hampshireosis
- Species: Blue-spotted salamander
- Breeding period & egg deposition: March to April
- Time to hatching (days): 30
- Larval period: 60 to 80 days
- Metamorphosis: June through mid-August
- Comments: Vernal pool indicator species in New Hampshire
- Species: Spotted salamander
- Breeding period & egg deposition: March to April
- Time to hatching (days): 31 to 54
- Larval period: 60 to 110 days
- Metamorphosis: July to September
- Comments: Vernal pool indicator species in New Hampshire
- Species :Red-spotted newt
- Breeding period & egg deposition: April to June, August to October
- Time to hatching (days): 21 to 35
- Larval period: 60 to 90 days
- Metamorphosis:July to October
- Comments: Most larval newts transform into a terrestrial red eft stage that remain on land from 3 to 7 years before transforming into aquatic adults. Some populations skip the eft stage, remain in the water, and become adults in about 2 years.
- Species: Four-toed salamander
- Breeding period & egg deposition: March to May
- Time to hatching (days): 38 to 60
- Larval period: 42 to 126 days
- Metamorphosis: June to September
Frogs
- Species: Eastern American toad
- Breeding period & egg deposition: April to June
- Time to hatching (days): 3 to 12
- Larval period: 21 to 70 days
- Metamorphosis: June to July
- Species: Fowlers toad
- Breeding period & egg deposition: May to August
- Time to hatching (days): 7
- Larval period: 40 to 60 days
- Metamorphosis: July through August
- Species: Northern spring peeper
- Breeding period & egg deposition: March to June
- Time to hatching (days): 6 to 12
- Larval period: 90 to 100 days
- Metamorphosis: July
- Species:Gray tree frog
- Breeding period & egg deposition:May to July
- Time to hatching (days): 4 to 5
- Larval period: 50 to 60 days
- Metamorphosis: June through Septemberember
- Species: Bullfrog
- Breeding period & egg deposition: May to July
- Time to hatching (days): 5 to 20
- Larval period: 2 to 3 years
- Metamorphosis: June to August
- Species: Green frog
- Breeding period & egg deposition: April to August
- Time to hatching (days): 3 to 6
- Larval period: 1 to 2 years
- Metamorphosis: June to August
- Species: Mink frog
- Breeding period & egg deposition: June to August
- Time to hatching (days): unreported
- Larval period: 1 to 2 years
- Metamorphosis: July to Augusty
- Species: Wood frog
- Breeding period & egg deposition: March to May
- Time to hatching (days):10 to 30
- Larval period: 42 to 105 days
- Metamorphosis: June to July
- Comments: Vernal pool indicator species in New
Hampshire
- Species: Northern leopard frog
- Breeding period & egg deposition: March to May
- Time to hatching (days):13 to 30
- Larval period: 63 to 84 days
- Metamorphosis: August to September
- Species: Pickerel frog
- Breeding period & egg deposition: April to May
- Time to hatching (days): 11 to 21
- Larval period: 80 to 100
- Metamorphosis:July to September
From: DeGraaf, R.M., and M. Yamasaki 2001. New England Wildlife: habitat, natural history, and distribution. Univ. Press of New England. 482 pp.
At the breeding wetland, adults mate and females deposit their eggs in the water (See inset page 4). There, the eggs develop and hatch in anywhere from four days (e.g., American toads and gray tree frogs) to as many as 30 or more days (e.g., four-toed salamanders and spotted salamander). Eggs hatch into free-swimming larvae that are completely aquatic, breathing through gills like fish until they develop lungs and leave the wetland for land as juveniles (small frogs, toads, or salamanders).
The time required for larvae to metamorphose into their terrestrial form and leave the pond varies dramatically among amphibian species (Table 1). Gray tree frogs, American toads, and wood frogs have the shortest larval periods and may reach metamorphosis as quickly as 21 to 42 days after hatching. The larval period for spotted salamanders and northern spring peepers averages about 80 days. Two frog species have larval periods as long as or longer than one year: green frogs may remain tadpoles for one year or more and bullfrogs may remain tadpoles for about two years before reaching metamorphosis.
The importance of wetland hydroperiod how long the wetland holds water becomes immediately evident when we consider the larval period of some amphibian species. Very simply, if the natal pond dries before larvae reach metamorphosis, the larvae will die. Species with very long larval periods are excluded from breeding successfully in wetlands with short hydroperiods, because short-hydroperiod wetlands don't hold water long enough for the larvae to complete their development.
Wetlands occur along a hydrologic gradient
Each wetland has its own hydroperiod. In any given town a variety of different wetlands occur, each with its own hydroperiod, with many filling and draining on a yearly basis. Biologists consider each wetland to be located along a hypothetical hydrologic gradient that ranges from ephemeral ponds that contain water for only a few weeks during the year to permanent lakes and ponds that never dry up. We can group wetlands into three main hydroperiod categories:r development.
- Short hydroperiod: These are ephemeral wetlands that, after any ice melts, hold water for less than four months a year. These wetlands tend to dry by May, June or July of each year. Shorthydroperiod wetlands are also considered vernal pools. (See inset page 7)
- Intermediate hydroperiod: These are ephemeral wetlands that hold water for at least four months (post ice-out) and tend to dry in late-July or later, or they dry only in years with low precipitation, so in some years they may hold water year-round. Intermediate wetlands often function as "vernal pools".
- Long hydroperiod:These wetlands don't dry; they always hold water. They are also called "permanent" lakes or ponds.
These general hydroperiod categories aren't arbitrary, but are based, in part, on observed differences in the species of amphibians, aquatic invertebrates, and fish that tend to be present or absent from wetlands in each category.
Vernal Pools and Their Indicators
Vernal pools are wetlands with a seasonal cycle of flooding and drying. Some vernal pools flood in the springwith water from melting snow, rain or high groundwater and then typically dry by summers end. Other pools follow a similar yearly pattern of flooding and drying, but they fill with rain in autumn, hold water all winter and spring, and then dry by late summer. This annual drying cycle means that predators such as fish can't maintain viable populations within vernal pools. As a result, vernal pools provide important breeding habitat for a number of amphibian species.
In New Hampshire, woods frogs and salamanders such as spotted, blue-spotted, Jefferson's, and marbled salamanders prefer to breed in vernal pools because the risk of predation to adults and larvae is lower than in wetlands that hold water throughout the year. Although some of these species will breed in permanent wetlands, they are all closely associated with vernal pools and are considered indicator species of these habitats.
When they are present, fairy shrimp are probably the best indicators of vernal pools in New Hampshire. The eggs of these small crustaceans must dry and be re-submerged with water before they will hatch. Although fairy shrimp aren't found in every vernal pool, their presence indicates a wetland that has dried at least once within the previous year.







It's important to realize the hydroperiod of any given wetland can actually vary greatly from one year to the next, depending primarily on the amount of precipitation an area receives. In very dry years the wetland in your backyard may hold water for only a few weeks during the spring, but in very rainy years, that same wetland may hold water well into the summer. In other words, a wetland that normally functions as a short hydroperiod pond may function as anintermediate pond in years with abundant precipitation. In very dry years, an intermediate pond may function as a short hydroperiod pond.
The timing of precipitation is also critical for determining if a pond will provide amphibian breeding habitat in any given year. If a pond remains dry during the breeding
and egg-laying period for any amphibian species, that pond will likely not provide breeding habitat for those amphibians that year, regardless if conditions change and the pool fills later in the season. As we discuss later, these yearly differences in wetland hydroperiod can result in actual differences in the species of amphibians and aquatic insects that use or can successfully breed in any pond from one year to the next.
Separating wetlands into short-, intermediate, and longhydroperiod categories requires visiting each wetland a minimum of three times within a single year (e.g., mid- May, late-June, early-August). The maximum depth of water present during each visit, combined with other site indicators, such as vegetation and the presence of certain amphibian larvae and aquatic insects, provides a relatively accurate means to assess or predict a wetland's hydroperiod for the current year.
Figure 1. Difference in relative abundance of pond breeding
amphibians across the hydroperiod gradient.
(ADD FIGURE)
Table 2. Wetland hydroperiod categories and New Hampshire
amphibians and predacious aquatic insects associated with each.
An asterisk indicates the species can be found within wetlands
of that hydroperiod category. An "x" indicates the hydroperiod
category in which the species occurs in the greatest abundance.
(ADD TABLE)
How hydroperiod affects amphibian use of wetlands
When we look at short-, intermediate-, and longhydroperiod wetlands we find differences in both the numbers of amphibian species and the number of individuals of each species present.(Fig. 1, Table 2). These differences in amphibian presence and abundance result mainly from the ability of each species to cope with different drying times and levels of predation risk across the hydrologic gradient (Table 3).
Role of amphibian larval period
Differences in the length of the amphibian larval period is a main factor influencing which amphibian species occur or breed successfully in short-, intermediate-, and
long-hydroperiod wetlands. Basically, amphibians with long larval periods require ponds with a long hydroperiod. Amphibians that breed in ephemeral wetlands (i.e., short and intermediate hydroperiod) usually have shorter larval periods; a shortened larval period is considered an adaptation that allows some species to better cope with a short hydroperiod.
Table 3. A comparison of factors influencing the suitability of short, intermediate, and permanent hydroperiod wetlands
in providing habitat for pond-breeding amphibians in New Hampshire.
(ADD TABLE)
For example, wood frogs and spotted salamanders, whose larval periods are as short as 42 and 60 days respectively, commonly breed in short- and intermediate-hydroperiod wetlands. These species deposit eggs in the spring, the eggs hatch, and the larvae develop and metamorphose into juveniles able to leave wetlands that dry as early as June (wood frogs) or July (spotted salamanders).
In comparison, green frogs and bullfrogs, whose tadpoles require at least one full year to develop, breed most commonly in long-hydroperiod wetlands. These species deposit eggs in the summer, the eggs hatch and the tadpoles develop, overwinter in the pond, and metamorphose into juveniles either one year (green frogs) or two years (bullfrogs) later.
Most amphibian species can be found in more than one wetland hydroperiod category, but they tend to occur in their greatest numbers in wetlands of a certain hydroperiod (Table 2). For example, wood frogs breed in both short- and intermediate-hydroperiod wetlands; however, they are most abundant in intermediate wetlands. This is explained, in part, by the fact that intermediate-hydroperiod wetlands provide the best opportunity for wood frogs to complete their metamorphosis most years.

body and dark brown “mask” behind the eye.

are laid by an individual female, while the large
mass (left) is a collection of individual
masses laid by multiple females.
During very dry years, short hydroperiod wetlands may never fill with water or they may dry before wood frog larvae can metamorphose and escape the pond. By contrast, intermediate wetlands may hold water long enough during very rainy years for green frog tadpoles to overwinter and metamorphose the following year. However, green frogs tend to be most abundant in long-hydroperiod wetlands; a permanent hydroperiod wetland offers much more predictable habitat for a species that requires typically more than one year to metamorphose.
When considering the required larval period of each amphibian species (Table 1), it's easy to understand why bullfrogs and green frogs don't occur in short hydroperiod wetlands. But why are wood frogs usually absent from permanent wetlands? The answer has to do with the risk of predation, and the fact that larval amphibians are exposed to a wide range of predators across the hydrologic gradient. Differences in the type and abundance of predators found in short-, intermediate-, and long-hydroperiod wetlands significantly influence where each amphibian species can breed most successfully.
Role of aquatic predators
The hydroperiod of a wetland determines not only how much time larval amphibians have for developing to metamorphosis, but also the type and number of predators they may encounter. Fish, aquatic insects, and redspotted newts are among the most important predators of amphibian larvae and eggs. Each of these predators occurs in different abundance in short-, intermediate-, and long-hydroperiod wetlands. These differences in predator distribution influence which wetlands provide suitable breeding habitat for amphibians. As a general rule, the number of predator species and the overall abundance of predators increase as we move from short- to long-hydroperiod wetlands (Table 3).
Fish are by far the most important predators of larval amphibians. Fish can significantly reduce or completely eliminate tadpoles and small salamanders from wetlands. Although fish are occasionally found in ephemeral wetlands (e.g., ponds occasionally flooded with water containing fish), they occur most commonly in permanent ponds. As a result, some amphibian species (e.g. wood frogs) almost completely avoid breeding in permanent ponds because they can't co-exist with fish.
Other species such as spotted salamanders will breed in permanent ponds, but their breeding success is greater in ephemeral wetlands that lack fish. As a result, short- and intermediate-hydroperiod wetlands that lack fish provide especially important breeding habitat for a number of our pondbreeding amphibians (e.g., wood frogs, spotted salamanders, blue-spotted salamanders, marbled salamanders). As such, these wetlands are essential to the long-term maintenance of amphibian species diversity across our landscape.
Most people commonly think of frogs eating insects. However, many are surprised to learn a number of aquatic insects readily consume tadpoles and larval salamanders. In fact, after fish, aquatic insects are some of the most important predators of larval amphibians. However, unlike fish, predaceous aquatic insects occur regularly in wetlands within every hydroperiod category.

predators of larval amphibians. Wood frogs, spotted
salamanders, and blue-spotted salamanders
breed most successfully in ephemeral ponds,
which because they dry, are unlikely to support
viable populations of fish.

eggs and are often present in short-hydroperiod
wetlands.

provide habitat for a diversity of aquatic insects
that prey on amphibian larvae. Some, like this
diving beetle larvae (Dytiscidae), are capable of
capturing large tadpoles and small fish.
As we move up the hydrologic gradient, the abundance and number of predaceous insect species increases, as does their body size (Table 2).
This means predation from aquatic insects tends to be lowest in shorthydroperiod ponds. Here, amphibian larvae are exposed to only a few small species of predaceous diving beetles and a few small species of dragonfly larvae. These insects are capable of capturing and eating small, free-swimming amphibian larvae. Caddisfly larvae are also present in shorthydroperiod ponds. These insects prey primarily on frog and salamander eggs.
In intermediate- and long-hydroperiod wetlands, amphibians are exposed to an ever-greater abundance of large predaceous insects, including giant water bugs, large dragonfly larvae, water scorpions, and a diversity of predaceous diving beetles. These insects consume frog and salamander larvae. In fact, some of these insects are large enough to even capture and kill small fish and large bullfrog tadpoles. The threat of predation by aquatic insects is greatest for amphibian larvae in long-hydroperiod ponds.
Similar to aquatic insects, other predators such as red-spotted newts can be found in wetlands in every hydroperiod category (Table 2). Adult and larval newts will readily eat any tadpole or salamander larvae they can fit in their mouths. As a result, newts can significantly influence the survival rates of other amphibians. Although newts are occasionally found in shorthy droperiod wetlands, they are most common and can only maintain populations in wetlands that don't dry annually (Table 2).

of every hydroperiod category. Adult and larval
newts readily prey on the eggs and larvae of other
amphibians. These adult newts are eating wood
frog eggs.


large body size, making them less vulnerable to
predation from aquatic insects and small fish. Tadpoles
of bullfrogs and American toads also have
toxic skin that makes them taste bad to fish.
Amphibian defenses against predators
Amphibians aren't entirely defenseless against the predators trying to eat them. A basic, but effective, defense against predators is simple avoidance. Wood frogs are an example of a species whose best defense against predators is avoidance. Because wood frogs typically develop in short-hydroperiod wetlands, their larvae must feed constantly to reach
metamorphosis before the pond dries. Constant swimming and foraging by wood frog tadpoles makes them very conspicuous to predators such as fish, dragonfly larvae, and giant water bugs, which are attracted by the movement of their prey. As a result, wood frogs tend to avoid breeding in permanent ponds where these predators are most common and abundant.
Amphibian species with long larval periods don't have the luxury of being able to avoid permanent wetlands, which often contain fish and abundant predatory aquatic insects. Species such as green frogs and bullfrogs have developed other strategies to avoid predators. One common strategy is to simply reduce their activity. Since bullfrogs and green frogs typically breed in permanent ponds, they can afford to lower their activity and feed less frequently than amphibians using ephemeral ponds. By reducing the amount of swimming and foraging they do, green frog and bullfrog tadpoles make themselves less vulnerable to predators that detect their prey by movement. In some cases, tadpoles of these species are able to detect predators by sight, vibration, or chemical cues and stop swimming to avoid detection until the predator passes by.
Bullfrogs and green frogs have a few other important defenses that protect them against predators. Both species attain large body size as tadpoles, making them less vulnerable to predation from fish and dragonfly larvae that are limited by how widely they can open their mouths. However, even the largest tadpoles can still be vulnerable to large fish or to aquatic insects such as giant water bugs, which inject toxins into their prey and have sucking mouthparts rather than chewing jaws. One last
defense possessed by bullfrogs, green frogs, and American toads is a toxic skin making them taste bad to some fish.
Some amphibians such as spring peepers have larvae with a high degree of foraging activity, relatively small body size, and little if any chemical defenses against predators, yet they are still able to breed successfully in permanent wetlands with fish and abundant aquatic insects. The success of these amphibians in permanent ponds is based on their use of densely-vegetated microhabitats where larvae can escape from predators. Such a strategy isn't entirely effective, but it does allow some larvae to survive to metamorphosis.
Which wetlands support the greatest diversity of amphibian species?
Table 2 reveals that short-hydroperiod wetlands are used by only a small number of amphibian species. This is because short-hydroperiod wetlands don't hold water long enough for larvae of most amphibian species to metamorphose before the pond dries, and they can be very ephemeral during years when precipitation is low.
These characteristics make short-hydroperiod wetlands suitable for only those species with the fastest larval development rates (e.g., American toads). This is especially true for wetlands with hydroperiods less than four months long.
In comparison, long-hydroperiod wetlands hold water long enough for all amphibians to reach metamorphosis, but they also support the greatest diversity and abundance of predators (Table 2). As a result, long-hydroperiod wetlands are the best breeding habitat for only those species with good predator defenses and/or larval periods
greater than one year (e.g., bullfrogs, green frogs, redspotted newts).
Wetlands with an intermediate hydroperiod offer many amphibians a suitable balance between wetland permanence and risk of predation. During most years, intermediate hydroperiod wetlands hold water long enough for most amphibian species to escape the pond before it dries. Also, because intermediate wetlands dry periodically, they typically don't support fish and they contain fewer predacious aquatic insects. As a result, intermediate wetlands tend to support the greatest diversity and abundance of amphibians, as compared to short- and long-hydroperiod wetlands.
Maintaining amphibian diversity
Natural resource professionals and community planners often have the responsibility of minimizing the impacts of development on wetland resources. Unfortunately, it's not always possible to avoid loss or alteration of individual wetlands during some development projects. In these situations, professionals and planners have the difficult task of deciding which wetlands are most valuable and worthy of protection, and which ones the community can lose or alter. Many wetland functions and values deserve consideration when making these decisions.
One function to consider is wetlands' wildlife habitat (others include ecological integrity, flood control, groundwater use, sediment trapping, and historical value). The remainder of this article explains how knowledge of wetland hydroperiod can help determine which wetlands within a community are most valuable for maintaining functioning, diverse populations of pond-breeding amphibians.
Maintain wetlands of every hydroperiod
Amphibian species aren't distributed randomly across the hydrologic gradient, but rather, occur in wetlands that provide them the best combination of habitat permanence and lowest risk of predation. Therefore, if the goal is to protect and maintain amphibian biodiversity, a diversity of wetlands with different hydroperiods must be protected and maintained.
To accomplish this goal, communities must make an attempt to identify and broadly classify wetlands within a community by their hydroperiod (i.e., short, intermediate,
long). Doing so allows natural resource professionals and community planners to minimize impacts to wetland types underrepresented on the local landscape. For example, if short-hydroperiod wetlands are rare within a proposed project area, focusing development impacts on wetlands with longer hydroperiods may be less detrimental to local amphibian diversity.
A general, but effective approach would be to maintain a variety of natural ponds with hydroperiods ranging from as little as 30 days to those with hydroperiods of at least three years. Such an approach will provide breeding habitat for the majority of pond-breeding frogs and salamanders (as well as aquatic invertebrates) and help to ensure some breeding success even in very dry or very wet years. To apply this approach, a community must first identify a method to accurately identify wetland hydroperiod in the field and then answer the question of how many wetlands must be protected within an area to maintain viable populations of pond-breeding amphibians.
Figure 2. Differences in the presence/absence of vernal pool-associated amphibians and aquatic invertebrates due to yearly
variability in wetland hydroperiod.
(ADD FIGURE)
Identifying wetland hydroperiod
The most difficult aspect of classifying wetlands by their hydroperiod is that the hydroperiod of each wetland can vary from one year to the next, due mainly to changes in precipitation. Because of this, differences in a wetland's hydroperiod can result in real differences in the species of amphibians and aquatic invertebrates found in that wetland from year to year.
To illistrate this point, follow "Wetland A" over the course of two consecutive years to see how changes in yearly precipitation influence its hydroperiod and the presence/absence of amphibians and aquatic invertebrates supported by the wetland (Fig. 2).
- In Year One, "Wetland A" fills with water in early-March (due to ice-melt and precipitation) and the wetland doesn't dry until late-July. This year Wetland A functions as an intermediate hydroperiod wetland, holding water for nearly five consecutive months. Spotted salamanders and wood frogs are abundant and larvae are able to complete their development and emerge from the wetland before it dries. Fairy shrimp are present. Caddisfly larvae, diving beetle larvae in the genera Acilius and Dytiscus, and larvae of dragonflies in the genus Sympetrum are all present (Fig. 2).
- In Year Two there is a drought; Wetland A doesn't fill with water until mid-May and it dries completely by mid-June. During this year Wetlandm A functions as a short hydroperiod wetland, holding water for less than 2 months. Wood frogs do not breed in Wetland A in Year Two because the wetland does not fill with water until after the breeding season. Spotted salamanders are not observed here in Year Two, and any eggs that might have been deposited here could not have hatched before the wetland dries. Fairy shrimp are not present this year. Caddisfly larvae and larval Acilius spp. are present but larval Dytiscus spp. and Sympetrum spp. are not.
Although the wetland in Figure 2 is a hypothetical example, it's based on the results of a University of New Hampshire study and on actual observations of wetlands in southern New Hampshire. This example illustrates the variability a single wetland can exhibit in hydroperiod and amphibian species presence/absence from year to year. Most importantly, it points to potential errors that can arise when relying solely on single-year and especially single-visit assessments of wetlands when trying to determine their hydroperiod and use by amphibians.
During most years the wetland in Figure 2 has an intermediate hydroperiod and provides breeding habitat for spotted salamanders, wood frogs, and fairy shrimp (all three are indicator species for vernal pools in New Hamsphire). However, if this wetland was assessed only during Year Two, and the wetland assessor had no prior knowledge of this wetland, the assessor may conclude that the wetland doesn't hold water long enough to provide functional breeding habitat for amphibians; consequently, the wetland may be immediately deemed appropriate for disturbance.
The yearly variability in wetland hydroperiod described above is natural for ephemeral wetlands (i.e., short- and intermediate hydroperiod). Even in wetlands within "pristine" or "undisturbed" landscapes, amphibians may occasionally experience complete reproductive failures due to yearly differences in precipitation. Such variability is inherent in these wetlands and the amphibian species that use them and does not, in itself, make these wetlands any less valuable as amphibian breeding habitat.
Suggestions for assessing wetland hydroperiod
It's very difficult to assess hydroperiod accurately from a single visit to a wetland. Such assessments provide only a snapshot of how the wetland actually functions in the landscape in providing breeding habitat for amphibians. Ideally, wetland hydroperiod and use by amphibians should be assessed with multiple-year visits to the same wetland, with at least one year of visits conducted during a year when precipitation is similar to the average for the region in which the wetland occurs.
So, just how many years should a wetland be visited to provide an accurate assessment of how it functions? Basically, the more years of data you can collect from a single wetland the better you'll understand the typical hydroperiod of that wetland and what amphibian species use it. Two years of data are better than one; three years of data are better than two, etc.
Unfortunately, it isn't practical in most situations to expect that wetlands within a proposed project area will be sampled over several successive years. Financial constraints and project and permit deadlines typically require that all wetlands within a project area be assessed during a single year, and often only a single visit to each wetland can be conducted.
In these cases, vegetation within and around the wetland and the presence/absence of specific amphibians, aquatic invertebrates, and fish must be used to make the best prediction of each wetland's general hydroperiod. To be accurate, such assessments require a skilled biologist or wetland assessor with the ability to identify amphibian egg masses to species, tadpoles and salamander larvae to species, and aquatic invertebrates to at least the level of genus.
Assessments will require a thorough search of each wetland for egg masses, fairy shrimp, and fish, as well as dip-netting to capture amphibian larvae and aquatic insects. Single-visit assessments should be used only to separate ephemeral wetlands (i.e., those that typically dry during the year) from those with more permanent hydroperiods. Separating wetlands into short-, intermediate, and long-hydroperiod categories requires a minimum of three visits to each wetland within a single year (e.g., mid-May, late June, early August). Noting the maximum depth of water present during each visit and combining this measurement with the following site indicators can provide a relatively accurate means to assess or predict a wetland hydroperiod for the current year.
Site indicators for predicting wetland hydroperiod
Vegetation growing within and around a wetland provides a simple and relatively accurate indicator of general wetland hydroperiod (e.g., ephemeral or permanent). Research of wetlands in Rhode Island showed that ponds with hydroperiods less than nine months long had more shrub species growing around their perimeters and greater shrub coverage (average was 18 percent coverage) within the ponds, as compared to wetlands with longer hydroperiods. Shrubs common around the perimeters of ephemeral ponds included highbush blueberry (Vaccinium), sweet pepperbush (Clethra), and swamp azalea (Rhododendron). Shrubs common within ephemeral ponds included buttonbush (Cephalanthus), Steeplebush (Spirea), and winterberry holly (Ilex). Conversely, the presence of floating aquatic plants such as pondweed (Potamogeton), water milfoil (Myriophyllum), and water lily (Nuphar and Nymphaea), and/or persistent herbaceous vegetation such as cattail (Typha), pickerel weed (Pontederia), and arrowhead (Sagittaria) often indicate ponds with more permanent hydroperiods.
The presence of specific amphibian larvae and aquatic invertebrates also can be used to assess whether a wetland tends to dry during the year or whether its hydroperiod is more permanent. Fairy shrimp are probably the best indicator of wetlands that dry at least periodically, because fairy shrimp eggs must dry and be re-submerged to hatch. Wood frog eggs and wood frog tadpoles also are generally reliable indicators of ponds that dry annually; wood frogs tend to avoid depositing their eggs in permanent ponds due to their inability to cope with fish and large predaceous aquatic insects. Caution should be used, however, because wood frogs will occasionally breed in permanent ponds that lack fish (e.g., dug farm ponds).
A variety of organisms can be used to identify wetlands that hold water for at least one year. Although fish can occasionally be found in ephemeral wetlands, they are most common in permanent ponds. The position of a wetland on the landscape can often be helpful in determining if fish have been introduced to a pond.
Ephemeral ponds in river floodplains and those within or adjacent to intermittent streams are prone to periodic fish introductions during periods of high water flow. A few amphibians can serve as indicators of longer hydroperiod wetlands. For example, the presence of bullfrog and green frog tadpoles at least one year old is a good indication a wetland didn't dry completely the previous year.
Finally, a number of aquatic insects are found primarily in wetlands that hold water for at least one year. These include dragonfly larvae of the genera Aeshna, Cordulia, Anax, Erythemis, Libellula, and Pachydiplax. Additionally, giant water bugs (Lethocerus and Belostoma), waterscorpions (Ranatra), and creeping water bugs (Pelocoris) are most common in longer-hydroperiod wetlands. It's important to note that accurate identification of these insects and most tadpoles in the field requires considerable practice and experience.


as water lily (Nuphar and Nymphaea) and
pondweed (Potamogeton), and/or persistent
emergent plants such as cattail (Typha), pickerel
weed (Pontederia) or arrowhead (Sagittaria) often
indicate ponds with permanent hydroperiods.
When to conduct hydroperiod assessments
Late April to mid-May is an ideal time to conduct a one-time assessment of wetlands to predict hydroperiod and use by amphibians. Usually by this time fairy shrimp, wood frogs eggs or tadpoles, and eggs of spotted salamanders and blue-spotted salamanders of the current breeding season can be found within the wetland. Tadpoles of bullfrogs and green frogs that
may have over-wintered in the pond also should be present. Additionally, larvae and adults of the above mentioned aquatic insects can generally be found in wetlands by this time if they are going to be present.
A few words of caution when conducting wetland assessments: First, the presence of adult frogs within a wetland should be used as an indicator of breeding activity only if the frogs are also heard calling, or seen mating or depositing eggs. Second, little can be concluded from the absence of amphibians during a single-visit assessment of a wetland. Breeding efforts of amphibians vary from year to year and slight changes in environmental conditions such as temperature and precipitation can cause some species to either delay or completely forgo breeding if conditions aren't favorable.
Finally, the suggested assessment dates provided above are most appropriate for southern New Hampshire, from the Massachusetts border to the Lakes Region. Assessments south of this region should be conducted slightly earlier and those to the north slightly later to account for differences in average temperatures that affect amphibian breeding dates and emergence of aquatic invertebrates.
How many wetlands must be protected?
When deciding which wetlands are most important for protection and which ones can be lost or altered with the least impact to amphibians, the question arises of just how many wetlands you need to protect. Unfortunately, we currently don't know what percentage or minimum number of ponds within any given area must be protected, or what configuration of ponds is needed, to maintain viable populations of pondbreeding amphibians.
We do know, however, that loss or alteration of any wetland reduces the total number of sites at which pond-breeding amphibians can reproduce and/or use as steppingstones for dispersal. Therefore, avoid or at least minimize the loss or alteration of wetlands whenever possible. The following are suggestions for minimizing impacts to pond-breeding amphibians in situations where wetland loss or disturbance is unavoidable.


Conserving wetlands across the landscap
- To conserve the greatest diversity of pond-breeding amphibians, take a landscape approach to conserving a diversity of wetlands that span the hydrologic gradient, and consider providing greater protection to those wetlands with hydroperiods unique or uncommon for your area. The following points may be helpful in determining which wetlands within an area are most suitable for increased protection:
- Wetlands inundated for less than four months are functional and important components of the landscape. These wetlands may provide critical breeding habitat for fairy shrimp and may support wood frogs, spring peepers and American toads. During years when precipitation is high these wetland may support a greater diversity of amphibian species. These wetlands may also function as steppingstones for amphibians dispersing to new habitats.
- Wetlands with hydroperiods at least four months long are required by the majority of pond-breeding amphibians.
- Wetlands with hydroperiods between four and 11 months long are especially important for supporting the widest diversity of amphibians and for protecting against complete reproductive failures during years with low precipitation.
- Wetlands without inlets or outlets may be especially important to pond-breeding amphibians, because they are less likely to support fish.
- In New England, all else being equal, modifying wetlands with hydroperiods less than four months long may have less impact on amphibian species diversity than modifying non-permanent wetlands with longer hydroperiods.
Wetland size is a poor indicator of importance
A wetland's size is a poor indicator of its importance as amphibian breeding habitat. Researchers investigating wetland hydroperiod in wetlands in New Hampshire, Maine, and Rhode Island found no correlation between wetland size and the number of amphibian egg masses within wetlands, suggesting that small (less than one-half acre) ponds may be just as productive as larger ponds.
In fact, wetlands as small as one-tenth acre can provide critical habitat for spotted salamanders and wood frogs. Therefore, small ponds shouldn't be automatically associated with low value for wildlife. Hydroperiod is a much better indicator of wetland productivity than is wetland size. Furthermore, small ponds can play an important role in amphibian dispersal and contribute to the long-term stability of local amphibian populations.
Importance of amphibian dispersalof importance
Long-term success of local amphibian populations is dependant on their ability to exchange genetic material with other populations and to disperse across the landscape to colonize new habitats or to re-colonize populations that have gone extinct from a specific wetland.
Amphibians living in undisturbed habitats are naturally vulnerable to local extinctions (e.g., loss of all wood frogs
from a wetland) due to yearly variation in environmental conditions. Amphibians breeding in habitats where wetlands have been lost or hydroperiod has been altered are even more vulnerable to extinction.
Because adult amphibians have a strong tendency to return to the pond where they first started breeding (e.g., once a spotted salamander has bred in a pond it will return to that same pond to breed throughout its life), juveniles are the primary dispersers within a population. The ability of juvenile amphibians to find new habitats or to "rescue" extinct populations is strongly affected by the number of ponds in an area and the distance between ponds.
How far do juvenile amphibians disperse? Because juveniles of most amphibians are too small to track with conventional methods (i.e., radio tracking) we actually have very limited information on this aspect of amphibian life. For example, in Table 4, most of the studies cited are for work conducted on adults. What we do know is that juvenile amphibians are more susceptible to desiccation than larger adults, thus availability of multiple aquatic sites is an important component of good dispersal habitat. Wetlands of any size or hydroperiod may serve as steppingstones between viable habitats. In some situations, wetlands with hydroperiods less than four months long may serve as critical links between more viable habitats. In such cases, these short hydroperiod wetlands may be among the most important ones to protect on a given
Maintain intact uplands around and between wetlands
Efforts to protect individual wetlands without also protecting the uplands surrounding them won't successfully maintain viable amphibian populations. Uplands (i.e., non-wetland areas) provide required wintering and/or feeding habitat for many pond-breeding amphibians such as wood frogs, spring peepers, gray treefrogs, American toads, spotted salamanders, and bluespotted salamanders. In fact, adults of these species spend most of their lives in upland habitats. Although they require wetlands for breeding, they may spend only a few days to a few weeks each year in wetlands.
Additionally, uplands provide critical connections between wetland habitats. Amphibians require these upland connections during seasonal migrations to and from breeding wetlands, and by juveniles dispersing to new habitats. Protecting uplands should be considered a required part of protecting wetlands when the objective is to conserve populations of pond-breeding amphibians.
Unfortunately, we don't know what percentage of uplands around a wetland must be protected, or how far from a wetland disturbances must be kept to maintain functioning populations of these amphibian species. Because each site (e.g., a wetland and the uplands that surround it) differs from the next, protection measures that succeed in maintaining amphibians in one area may not be appropriate or effective at another.
For example, a 50-foot building setback from wetlands may be adequate for maintaining amphibian breeding, feeding, and dispersal habitat near one wetland, yet completely inadequate, or unduly restrictive at another. Fully evaluate the specific conditions of each project site, and consider the following to avoid or minimize the disturbance to amphibian habitat.
- Avoid disturbances such as the construction of buildings, roads, and driveways within 300 feet of wetlands whenever possible. Outside the breeding season, adults of many pond-breeding amphibians regularly use upland habitats between 50 to 300 feet away from the nearest wetland
- At a minimum, retain intact upland habitats between adjacent wetlands to provide suitable amphibian migration and dispersal routes.
- Retain cover objects such as leaf litter, surface stones, and fallen logs in corridors between adjacent wetlands and within 300 feet of all wetlands.
- Redirect new roads away from upland habitats between adjacent wetlands and at least 300 feet from wetlands when possible.
- When locating roads and driveways, avoid changes to surface water flow that will redirect water away from or into breeding wetlands. Such disturbances can alter the hydroperiod of affected wetlands.
- Avoid filling, ditching, draining, or deepening wetlands that provide functional amphibian breeding habitat.
- Avoid creating ruts in soils around wetlands during timber harvesting activities. Such disturbances can alter wetland hydrology and introduce sediment into wetlands which can interfere with the development of amphibian embryos and larvae.
Metapopulation
A metapopulation is essentially a population made of several populations that are spatially isolated and connected by periodic dispersal. In this example, five populations of different sizes (indicated by size number of frogs) and separated by varying distances represents a hypothetical metapopulation. Smaller populations have a higher probability of extinction. In (a) all sites have breeding frog populations, and in (b) a small population declines to zero. Dispersal from a neighboring population (c, d) results in "rescuing" the site and repopulation of the site through breeding (e). The circled population (f ) is both small and the most isolated. If that population were to decline to zero, "rescue" would be less likely.
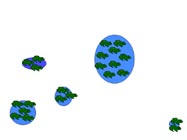

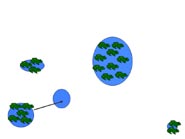
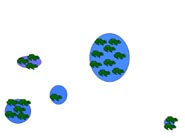
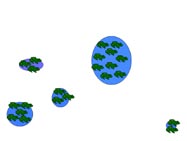
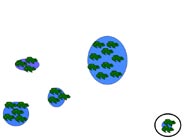
Table 4. Average and range of adult migration distance and metamorph/juvenile dispersal distance for three species of pondbreeding amphibians
(ADD TABLE)
Other Resources
 New Hampshire Wildlife Action Plan
New Hampshire Wildlife Action Plan
This plan provides New Hampshire decision-makers with important tools for restoring and maintaining critical habitats such as vernal pools, marsh and shrub wetlands, and floodplain forests, as well as populations of the state's wildlife species of conservation and management concern. The plan is available as a free download on the New Hampshire Fish and Game website at: http://www.wildlife.state.nh.us/wildlife/wildlife_plan.htm
 Identification and Documentation of Vernal Pools in New Hampshire
Identification and Documentation of Vernal Pools in New Hampshire
This guide, published by the New Hampshire Fish and Game Department's Nongame and Endangered Wildlife Program, explains vernal pools and their inhabitants. It is a crucial tool in documenting these important wildlife habitats in New Hampshire. This guide is available or purchase on the New Hampshire Fish and Game website at: http://www.wildlife.state.nh.us/shop/shop_books.htmn
Works Referenced:
Colburn, EA. 2004. Vernal Pools: Natural History and Conservation. Virginia, Blacksburg, The McDonald & Woodward
Publishing Company.
Babbitt, K.J. Unpublished data. 215 James Hall, University of New Hampshire, Durham, New Hampshire.
Babbitt, K.J. 2005. The relative importance of wetland size and hydroperiod for amphibians in southern New Hampshire,
USA. Wetlands Ecology and Management 13: 269-279.
Babbitt, K.J., M.J. Baber, and T.L. Tarr. 2003. Patterns of larval amphibian distribution along a wetland hydroperiod
gradient Canadian Journal of Zoology 81: 1539-1552.
Baldwin, R.F., A.J.K. Calhoun, and P.G. deMaynadier. 2006. Conservation planning for amphibian species with complex
habitat requirements: a case study using movements and habitat selection of the wood frog Rana sylvatica. Journal
of Herpetology 40: 442-453.
Bellis, E.D. 1965. Home range and movements of the wood frog in a northern bog. Ecology 46: 90-98.
Berven, K.A., and T.A. Grudzien. 1990. Dispersal in the woodfrog (Rana sylvatica): implications for genetic population
structure. Evolution 44: 2047-2058.
Brodie, E.D. Jr., and D.R. Fomanowicz, Jr. 1983. Prey size preference of predators: differential vulnerability of larval
amphibians. Herpetologica 39: 67-75.
Carr Research Laboratory, Inc. and Hyla Ecological Services, Inc. 2003. Conservation permit application package:
Algonquin Regional High School, Northborough, MA. (Telephone Carr Research Laboratory, Inc. (1-508-651-7027)
for more information).
Douglas, M.E., and B.L. Monroe. 1981. A comparative study of topographical orientation in Ambystoma (Amphibia:
Caudata). Copeia 1981: 460-463.
Faccio, S.D. 2003. Postbreeding emigration and habitat use by Jefferson and spotted salamanders in Vermont. Journal of
Herpetology 37: 479-489.
Freidenfelds, N.A. 2006. An experimental approach to understanding the impact of vernal pool buffer size on wood frogs
(Rana Sylvatica). M.S. thesis. University of New Hampshire. 83 pp.
Kleeberger, S.R., and J.K. Werner. 1983. Post-breeding migration and summer movement of Ambystoma maculatum.
Journal of Herpetology 17: 176-177.
Lawler, S. P. 1989. Behavioral responses to predators and predation risk in for species of larval anurans. Animal Behaviour
38: 1039-1047.
Madison, D.M. 1997. The emigration of radio-implanted spotted salamanders, Ambystoma maculatum. Journal of
Herpetology 31: 542-551.
McDonough, C., and P.W.C. Paton. 2007. Salamander dispersal across a forested landscape fragmented by a golf course.
Journal of Wildlife Management 71: 1163-1169.
Montieth, K.E., and P.W.C. Paton. 2006. Emigration behavior of spotted salamanders on golf courses in southern Rhode
Island. Journal of Herpetology 40: 195-205.
Pechmann, J. K., D. E. Scott, J. W. Gibbons, and R. D. Semlitsch. 1989. Influence of wetland hydroperiod on diversity and
abundance of metamorphosing juvenile amphibians. Wetlands. Ecology and Management 1: 3-11.
Regosin, J.V., B.S. Windmiller, R.N. Homan, and J.M. Reed. 2005. Variation in terrestrial habitat use by four pool-breeding
amphibian species. Journal of Wildlife Management 69: 1481-1493.
Rittenhouse, T.A.G., and R.D. Semlitsch. 2006. Grasslands as movement barriers for a forest-associated salamander:
migration behavior of adult and juvenile salamanders at a distinct habitat edge. Biological Conservation 131: 14-22.
Rittenhouse, T.A.G. Unpublished data. 212 Tucker Hall, University of Missouri, Columbia, Missouri.
Semlitsch, R.D. 1987. Interactions between fish and salamander larvae. Costs of predator avoidance or competition?
Oecologia 72: 481-486
Semlitsch, R.D. 1998. Biological delineation of terrestrial buffer zones for pond-breeding salamanders. Conservation
Biology 12:1113-1119
Skelly, D.K. 1995. A behavioral trade-off and its consequences for the distribution of Pseudacris tree frog larvae. Ecology
76: 150-162
Skelly, D.K. 1996. Pond drying, predators and the distribution of Pseudacris tadpoles. Copeia 1996: 599-605.
Skelly, D.K., and E. E. Werner. 1990. Behavioural and life-historical responses of larval American toads to an odonate
predator. Ecology 71: 2313-2322.
Tarr, T.L. 2000. Patterns of larval amphibian and aquatic insect distribution: effects of hydroperiod. M.S. Thesis. University
of New Hampshire. 100pp.
Tarr, T.L, M.J. Baber, and K.J. Babbitt. 2005. Macroinvertebrate community structure across a wetland hydroperiod
gradient in southern New Hampshire, USA. Wetlands Ecology and Management. 13: 321-334.
Travis, J., W.H. Keen, and J. Juilianna. 1985. The role of relative body size in a predator-prey relationship between
dragonfly naiads and larval anurans. Oikos 45: 59-65.
Vasconselos, D., and A.J.K. Calhoun. 2004. Movement patterns of adult and juvenile Rana sylvatica (LeConte) and
Ambystoma maculatum (Shaw) in three restored seasonal pools in Maine. Journal of Herpetology 38: 551-561.
Veysey, J.S. 2006. Effects of forest clear cutting on spotted salamander (Ambystoma maculatum) migration. M.S. Thesis.
University of New Hampshire. 91pp.
Wacasey, J.W. 1961. An ecological study of two sympatric species of salamanders, Ambystoma maculatum and Ambystoma
jeffersonianum, in southern Michigan. Doctoral dissertation, University of Michigan, Ann Arbor, Michigan.
Wellborn, G.A., D.K. Skelly and E.E. Werner. 1996. Mechanisms creating community structure across a freshwater habitat
gradient. Annual Review of Ecology and Systematics 27: 337-363.
Williams, P.K. 1973. Seasonal movements and population dynamics of four sympatric mole salamanders, genus
Ambystoma. Doctoral dissertation, Indiana University, Indiana.
Windmiller, B.S. 1996. The Pond, the forest, and the city: spotted salamander ecology and conservation in a humandominated
landscape. Doctoral dissertation, Tufts University, Medford, Massachusetts.m
About the authors
Matt Tarr grew up in Hancock, New Hampshire and his outdoor experiences as a youth led him to pursue degrees in both Wildlife Ecology and Forestry at the University of New Hampshire. After completing graduate school at UNH, Matt worked as a consulting forester and apprentice wetland scientist where he gained experience assessing and managing wildlife habitat and forest resources on private and community owned lands. In 2002 Matt came to work with UNH Cooperative Extension and he served five years as the Extension Resources Forest Educator in Rockingham County. Since January 2007 Matt has worked as the statewide Wildlife Specialist for UNH Cooperative Extension where he is responsible for developing educational programs about wildlife habitat management and conservation for natural resource professionals, landowners, communities and volunteers. Matt works closely with the New Hampshire Fish and Game Department providing educational and technical assistance to landowners and communities interested in managing and conserving wildlife habitat on their land.
Kim Babbitt is a professor of wildlife ecology in the Department of Natural Resources at the University of New Hampshire. As a behavioral and community ecologist, her research ranges from predator-prey interactions, toxicology, and tadpole behavior to effects of landscape changes on amphibian populations. She has examined aspects of land use change in agricultural, industrial forest, and suburban landscapes. She has authored or co-authored over 50 publications and technical reports and is the co-editor of Amphibians and Reptiles: Status and Conservation in Florida published in 2005.

