A guide for dairy farmers
Introduction
Milk quality impacts nearly every aspect of a dairy farm. Troubleshooting milk quality issues requires a whole farm approach, including nutrition and feed management, environmental cleanliness, milking parlor prep procedures
and maintenance, genetics, and even culling decisions. Setting goals and developing a milk quality program that focuses on prevention and targeted treatment strategies can help improve milk quality and reduce somatic cell counts.
While this factsheet reviews general guidelines for improving milk quality, be sure to check out the National Mastitis Council website for in-depth protocols, guidelines, and procedures.
Housing Environment and Nutrition
Maintaining a clean, dry environment is essential to minimize the risk of environmental pathogens and mastitis infections.
- Bedding type and management can prevent mastitis on dairy farms. Bedding should be topped off at least weekly and as needed. Although inorganic bedding (sand) is less supportive of bacterial growth than organic sources (straw and shavings), bacterial growth can occur in either bedding type if not appropriately managed. Collect a bedding sample and test for environmental pathogens if necessary.
- Provide sufficient ventilation. Mechanical ventilation may be necessary, particularly during warmer months.
- Alleys should be kept clean, and manure should be removed regularly to preventsplatter onto the udder. Stalls should be cleaned daily to remove any soiled bedding as cows can spend 12 to 14 hours per day lying and resting. If bedding is not clean and dry, this can present a risk of exposing teat ends to environmental pathogens1, particularly after milking when the teat canal may still be open.
Figure 1: Clean layer of shavings in tie-stall barn with mattresses.

Feed management can also help reduce herd somatic cell count (SCC).
- Deliver fresh feed and push up often. Fresh feed after milking has been shown to increase the amount of time cows spend eating after returning from the parlor2. This means that the cow is standing and eating after milking, giving more time for the teat canal to close before contacting potential bedding contaminants, and reducing the risk of mastitis.
- Nutrition plays a role in immunity. When dairy cows are in a negative energy balance, their immune system may be compromised and increase the risk of developing mastitis3. Manymetabolic diseases are also risk factors for increased SCC and potential clinical mastitis.
- Avoid overstocking pens, particularly pre-fresh and fresh pens. Adequate feed bunk space (recommended 24-30 inches per cow) can prevent competition and reduce the risk of metabolic disease and immune suppression.
- Mycotoxins may also negatively impact immune function, increasing the risk of infection4. Vitamin E5 and selenium6 supplementation have the potential to improve immune function, which may reduce SCC. However, supplements do not replace proper housing and feed management.
Milking Procedures
Proper milking procedures are essential for parlor efficiency, animal health, and quality milk production. Milking parlor protocols should be created to work with the cows’ biology with emphasis on consistency and proper timing of milking procedures7. Here are some additional tips on proper milking of lactating dairy animals.
- Handle cows in a low stress environment. Stress can prevent milk letdown, increase milking times, decrease milk yield, and compromise teat integrity.
- Wear disposable gloves and change them as needed to reduce the spread of pathogens to the teat.
- Strip cows prior to milking. This will stimulate milk letdown, remove the foremilk and allow you to check for milk consistency. A strip cup is a great tool to check for clots, coagulation, or other irregularities in milk, all of which are potential indicators of mastitis.
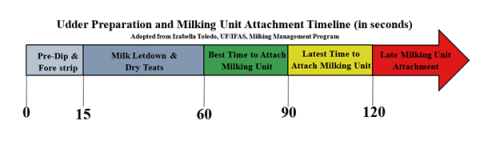
- Ensure proper timing (see Figure 2). Teat dip should be left on according to manufacturer directions (usually 30 seconds), and milking units should be attached 60-90 seconds after teat stimulation. This allows proper disinfection and ensures that the milking unit will be attached when oxytocin has stimulated milk letdown.
- If using automatic take-offs, avoid reattaching the milking unit unless it is kicked off. Overmilking cows can compromise teat integrity and increase risk of mastitis.
- You should not hear any squawking or air leaks. These are signs that the milking unit is not attached properly, and air is entering the claw, which can increase the risk of mastitis.
Figure 2. Udder preparation and milking unit attachment timeline.
Cleaning and Maintenance of Milking Equipment
Proper cleaning, sanitizing and routine maintenance of milking equipment is essential.
While we sometimes focus primarily on the in-parlor equipment, everything that comes into contact with milk is considered part of the milking system. Therefore, cracks in hoses, pulsator frequency, milk storage tanks and other maintenance issues can compromise animal health and increase SCC.
- Conduct maintenance at the recommended intervals. This includes, but is not limited to, routine replacement of hoses and clawgaskets (at least every 6 months), monitoring pulsators (rebuild at least 1-2 times per year) and vacuum levels, and preventative cleaning of drains, filters, and cleaning systems8.
- Monitor time, water temperature and pH during rinse (100ºF to 110ºF), wash (160ºF and 170ºF and pH between 11 and 13), post- rinse (100ºF to 110ºF and pH is between 3 and 4) and sanitizing (100ºF to 110ºF) cycles.Improper temperature and pH during cleaning procedures may result in buildup or residual films9.
- Check your water. Make sure to test water for bacteria or minerals and calculate the volume of water needed to clean the system, the water pressure, and flow rates10.
Mastitis Testing and Milk Culturing
The California Mastitis Test is a great tool to have on the farm for detecting mastitis. This tool can be used to identify inflamed quarters; however, you must perform a culture test to identify the specific type of mastitis pathogens.
Figure 3: California Mastitis Test paddle with reagent.

Culturing allows farms to understand the root cause of mastitis infections to develop targeted treatment and adjust management strategies based on the source of infection. Bulk tank culturing may be used to assess environmental cleanliness or generalized udder health but may not be suitable as a diagnostic tool10. Culturing individual cows with clinical mastitis can advise treatment plans, potentially reducing the number of treatments needed and overall antibiotic use11. It is also possible that no bacterial growth is observed on a clinical cow’s culture. When
this happens, it indicates that the source of the infection has been cleared through the cow’s immune system12. Culturing subclinical cows with high SCC may also be used to identify cows that, while not displaying clinical symptoms, are causing rises in bulk tank SCC. Farms should consult their veterinarian with culture results to determine the best treatment.
It is important to follow recommended procedures when collecting milk culture samples to avoid sample contamination.
- Always wear clean gloves.
- Prep the teat end as normal. Samples should be taken after teat prep and prior to milking unit attachment.
- Wipe the teat with alcohol. A new alcohol wipe or alcohol-soaked cotton ball should be used on each teat end.
- Use a sterile, unopened milk vial when collecting the sample. Strip the teat until enough milk is collected. Avoid touching the teat end to the milk vial.
- All samples should be labeled with appropriate cow appropriate cow ID, date, and quarter the milk was collected from. Check your lab for sample label instructions.
- Samples should be placed immediately on ice and refrigerated until shipped to the lab. Check your lab for sample storage and shipping instructions.
Regional Diagnostic Laboratories:
New Hampshire Veterinary Diagnostic Laboratory, UMaine Diagnostic Lab, Regional Dairy One
Data Records and Economics
On average, the cost per case of clinical mastitis in early lactation is $444, and this is associated with milk production loss, treatment costs, premature culling, discarded milk and extra labor involved with caring for the dairy cow 13. Maintaining treatment records is critical for developing prevention strategies and decreasing the number of mastitis cases. A quadrant graph (Figure 4) from a data records management software can be printed to help you evaluate incidences of mastitis by visualizing the previous SCC versus current SCC14.
Reviewing data such as a DHI single test day hot sheet, or the DHI herd summary can help identify high SCC cows15. While we may be quick to cull the cow with the highest SCC in the herd, this decision should consider the history of the cow including any treatment records, previous test day results and trends in somatic cell score or SCC.
Figure 4. Graphic of previous SCC versus current SCC obtained from data records management software. Each lactating cow represents a point of the graph.

Summary
Addressing milk quality issues is a whole farm approach. Remember that training employees in correct procedures, maintaining cleanliness, ensuring proper timing, keeping records and implementing prevention strategies are all ways to improve milk quality.
References
1DeVries, T. J., M. G. Aarnoudse, H. W. Barkema, K. E. Leslie, and M. A. G. von Keyserlingk. 2012. Associations of dairy cow behavior, barn hygiene, cow hygiene, and risk of elevated somatic cell count. J. Dairy Sci. 95(10):5730-5739. http://dx.doi.org/ 10.3168/ jds.2012-5375
2DeVries T.J., S. Dufour, D.T. Scholl. 2010. Relationship between feeding strategy, lying behavior patterns, and incidence of intramammary infection in dairy cows. J. Dairy Sci. 93(5):1987-1997 https://doi.org/10.3168/ jds.2009-2692
3van Straten, M., M. Friger, and N. Y. Shpigel. 2009. Events of elevated somatic cell counts in high-producing dairy cows are associated with dairy body weight loss in early lactation. J. Dairy Sci. 92(9):4386-4394. https://doi. org/10.3168/jds.2009-2204
4Fink-Gremmels, J. 2008. The role of mycotoxins in the health and performance of dairy cows. 2008. Vet. J. 176(1):84-92. https://doi.org/10.1016/j.tvjl.2007.12.034
5Politis, I. 2012. Reevaluation of vitamin E supplementation on dairy cows: bioavailability, animal health and milk quality. Anim. 6(9):1427-1434. https:// doi.org/10.1017/S1751731112000225
6Kruze, J. A. Ceballos, H. Stryhn, A. Mella, R. Matamoros, P. A. Contreras, V. Leyan, and F. Wittwer. 2007. Somatic cell count in milk of selenium-supplemented dairy cows after an intramammary challenge with Staphylococcus aureus. J. Vet. Med. A Physiol. Pathol. Clin. Med. 54(9):478-483. https://doi.org/10.1111/j.1439-0442.2007.00999.x
7Erickson, P. S. 2021. Correct Milking Procedures. https:// extension.unh.edu/resource/correct-milking-procedures
8Royster, E, and G. Cramer. Best Practices: Care of Milking Equipment. https://extension.unh.edu/resource/correct- milking-procedures
9Heguy, J. 2011. How to properly clean milking equipment. https://www.agproud.com/articles/20344- how-to-properly-clean-milking-equipment
10Bartlett, P. C. G. Y. Miller, S. E. Lance, and L. E. Heider. 1991. Use of bulk tank and milk filter cultures in screening for Streptococcus agalactiae and coagulase-positive Staphylococci. J. Food Prot. 54(11):848-851. https://doi. org/10.4315/0362-028X-54.11.848
11Lago, A., S. M. Godden, R. Bey, P. L. Reugg, and K. Leslie. 2011. The selective treatment of clinical mastitis based on on-farm culture results: I. Effects on antibiotic use, milk withholding time, and short-term clinical and bacteriological outcomes. J. Dairy Sci. 94(9):4441-4456. https://doi.org/10.3168/jds.2010-4046
12Yutzy, A. 2023. On-Farm Culture: The Smart Approach to Clinical Mastitis Treatment. https://extension.psu.edu/on- farm-culture-the-smart-approach-to-clinical-mastitis- treatment
13Rollin, E., K. C. Dhuyvetter, and M. W. Overton. 2015. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 122(3):257-264. https://doi.org/10.1016/j.prevetmed.2015.11.006
14Haggarty, A., and D. M. Amaral-Phillips. Using DHI and PCDART Records to Evaluate Incidences of Mastitis. Accessed 2023. https://afs.ca.uky.edu/content/using-dhi- and-pcdart-records-evaluate-incidences-mastitis
15Bewley, J., M. Arnold, and D. M. Amaral-Phillips. Are you Using the DHI “Hot Sheet” to Manage your Herd Somatic Cell Count? https://afs.ca.uky.edu/dairy/are- you-using-dhi-hot-sheet-manage-your-herd-somatic- cell-count
Photo Credits
Figure 1: Clean layer of shavings in tie-stall barn with mattresses. Photo: Sarah Allen, UNH
Figure 2. Udder preparation and milking unit attachment timeline. Credit: Izabella Toledo, UF/IFAS
Figure 3: California Mastitis Test paddle with reagent. Photo: Patricia Henderson, UMaine.
Figure 4. Graphic of previous SCC versus current SCC obtained from data records management software. Each lactating cow represents a point of the graph. Adapted from Haggarty and Amaral-Phillips, University of Kentucky
Extension Services & Tools That Help NH Farmers Grow
Newsletters: Choose from our many newsletters for production agriculture
Receive Pest Text Alerts - Text UNHIPM to (866) 645-7010


