Tomato Spotted Wilt Virus (TSWV): a virus you should know and be able to identify!
by Kaitlyn Orde, Research Associate, Dept. of Agriculture, Nutrition & Food Systems, UNH
With the number of pests and pathogens posing challenges to crop production, the threat of viruses can easily take the back burner. However, viruses can cause irreversible damage and significant crop loss, especially when not identified early and contained.
Tomato Spotted Wilt Virus (TSWV) is one virus worth knowing. While we don’t have current loss estimates for the Northeast region, it has been documented on multiple farms in each of the last several years in New Hampshire; in some cases, causing significant plant and revenue loss. TSWV is a well-known virus in other regions of the country. In the state of Georgia, for example, it is estimated to cause approximately $9 million in losses in tomato and pepper each year, and growers on the west coast have reported losing up to 50% of tomato plants to the virus.
TSWV is often introduced on asymptomatic plants and then spreads to vegetable seedlings or other plants that were started by seed. One of the primary paths of introduction is on vegetatively-propagated ornamental plant material, but many weed species that grow under benches and outside of high tunnels and greenhouses, can also introduce the virus. These include (but not limited to) dandelion, lambs’ quarters, purslane, shepherd’s purse and white clover. TSWV has an extremely wide host range of many hundreds of plant species, including chrysanthemums, petunias, impatiens, nasturtium, begonia, tomato, pepper, eggplant, potato, lettuce, spinach, cucurbits, beans, and more.
In New Hampshire, all of the documented cases of TSWV have come from operations that mixed ornamental crops and vegetable seedlings. Virus spread to tomato and pepper are common, and particularly devastating. In one case, virus infected tomato plants were transplanted in the field and the virus spread to nearby pepper and potato fields. In another case, tomato seedlings became infected in the greenhouse but not properly diagnosed and were transplanted into the field, and plants remained mostly stunted and unproductive for the growing season.
Symptoms of the virus differ substantially depending on the plant species infected, but can include stunting, necrotic spots on the leaves and stems, necrotic spots that progress into the death of petioles and growing tips, wilting or cupping of leaves, leaf discoloration (including a bronzing on the top of leaves), concentric rings and spots on leaves and fruit, and petals with spots and/or line patterns. Some symptoms can mimic those caused by bacterial and fungal pathogens, or chemical injury. Photos 1-5 (credit K. Orde) show TSWV symptoms on pepper and tomato on a farm in New England. While the source of virus was unknown, plants were raised in a greenhouse facility where vegetable seedlings and external ornamental cuttings cohabitated. Scouting occurred regularly and chemical control measures were taken, but the greenhouse manager reported difficultly controlling the thrips population.

Photo 1. TSWV symptoms on pepper leaves (photo K. Orde).

Photo 2. TSWV symptoms on tomato leaves (photo K. Orde).

Photo 3. Stunting of tomato caused by TSWV (photo K. Orde).
TSWV is spread by thrips. While there are multiple species capable of carrying and even spreading the virus, the Western Flower Thrips (Frankliniella occidentalis) is considered the most (and perhaps only) efficient transmitter of the virus (photo 6 – credit Jack T. Reed, Mississippi State University, Bugwood.org). The Western Flower Thrips is very small (only about 1.2 to 1.4 mm in length) and light yellow to dark brown in color, and acquires the virus when during the larval stage only. The virus is persistent in the thrips, meaning – the virus remains in the pest and can be spread by the thrips for its entire life. However, females do not pass TSWV onto their progeny, so each generation of thrips must reacquire the virus from infected plant material.

Photo 6. Western flower thrips (Photo Jack T. Reed, bugwood.org)
While the fact that the virus is not passed onto offspring is helpful, controlling the Western Flower Thrips is particularly challenging because of its biological characteristics. See Figure 1 for an illustration of the infection cycle. Eggs are deposited directly into an infected plant, making them inaccessible. After which, they undergo two larval stages where larvae generally remain in the flower bud or foliage, where they can be well-protected from contact insecticides and many natural enemies. After the second larval stage, the pest then moves to the soil and pupates. While in the soil they do not feed and are again protected from insecticides that are aimed at the foliage (weed or crop). When the adults emerge with wings, they feed primarily on the flowers and terminal buds. Pesticide resistance is a documented problem with controlling trips chemically.
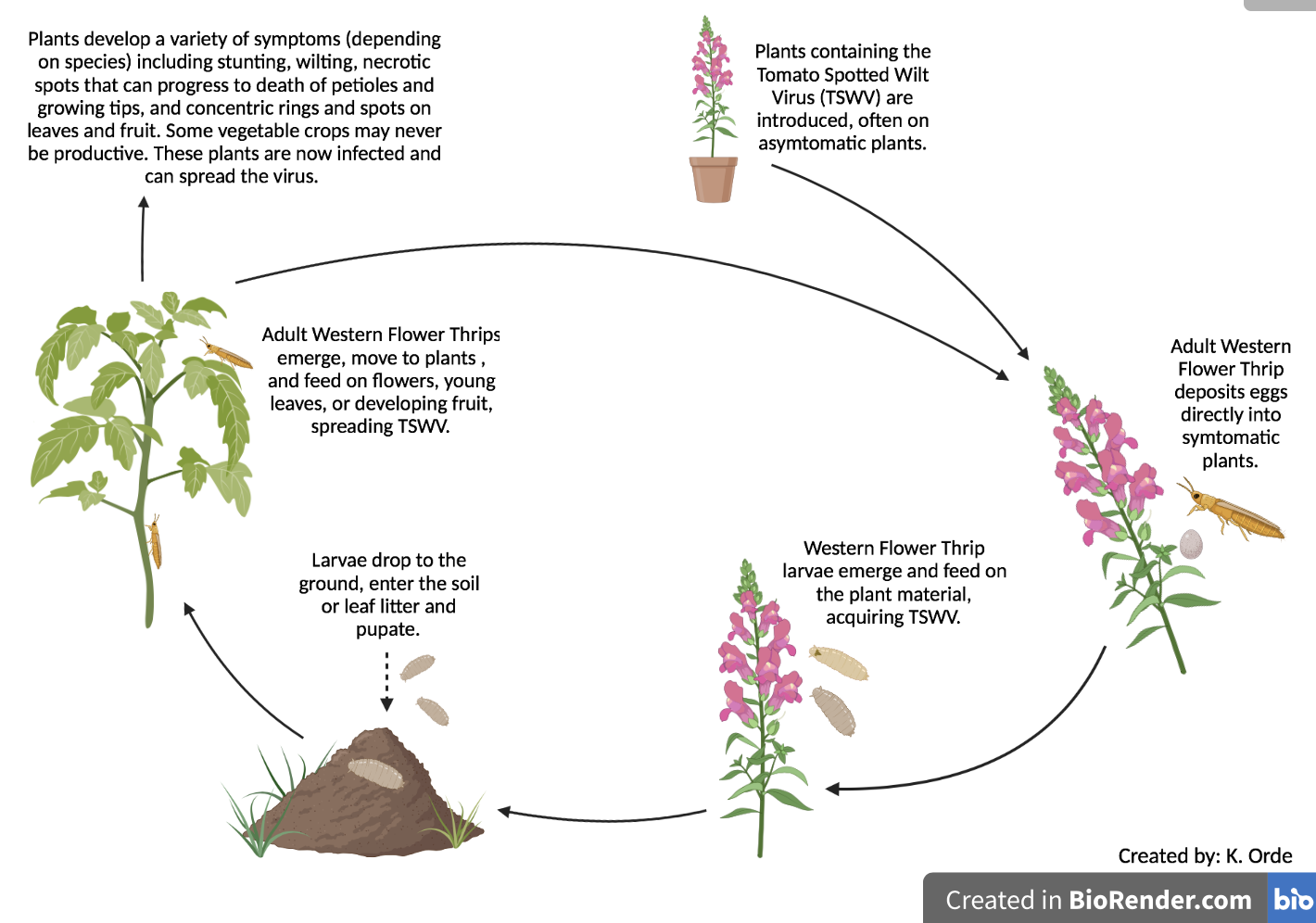
Figure 1. TSWV infection cycle (K. Orde).
The prevent and control the spread of TSWV, a mixture of physical, cultural, and chemical control measures may need to be taken. If infected material is present, TSWV cannot spread in plant populations if thrips are not present. Thus, the most obvious way to prevent the virus is to keep the thrips population under control. This can be difficult given how many thrips can be present in agricultural and greenhouse environments. Furthermore, thrips (including Western Flower Thrips) can overwinter in protective structures, particularly if there is landscape fabric or gravel present. Therefore, reducing the likelihood that infected material is introduced and present, is also important.
Using knowledge about your own operation and the tips we offer below, there are reasonable steps that can be taken to reduce risk. If you’ve had a TSWV outbreak or regularly battle thrips, consider how you can take steps this year to prevent another outbreak next year, and if there are updates to your current systems and operations that need to be improved to reduce pest pressure and TSWV risk.
Preventative
- Control weeds in and around greenhouses and fields. Weeds can harbor and introduce TSWV (especially after there has been an outbreak). They can also serve as habitat for thrips.
- Separate externally-sourced plant materials from that started on-site. In the Northeast, this often means keeping ornamental cuttings purchased from other nurseries separate from vegetable seedlings/transplants and other seed propagated crops that were started on-farm. We recognize it is common to intermix plants due to space limitations and cost-savings, but this cohabitation is highly risky due to TSWV’s wide host range and the likelihood that TSWV, thrips, or both are on imported material or somewhere on the farm.
- Try to obtain thrips and virus free plant material. ALWAYS inspect external plant materials for thrips (and other pests) upon delivery. Don’t be afraid to ask plant nurseries how they are preventing and testing for TSWV, if at all.
- Acquire TSWV field tests that can be run on-site to check for the virus. These field tests are only $5-$10 each (depending on quantity ordered), can be run in minutes, and are field tests meant to be used without any experience of equipment. They are already being used on farms with previous outbreaks and are a good tool for testing plant materials from external sources upon delivery. Be sure to order the correct tests, as there are different virus options. See Agdia.com for test kits: https://orders.agdia.com/agdia-immunostrip-for-tswv-isk-39300
- Purchase tolerant or resistant varieties when possible. For tomatoes and peppers, seek out varieties with the Sw-5b and Tsw genes, respectively. Resistance should be indicated in seed catalogues.
- Monitor for thrips using yellow or blue sticky cards placed at a density of 1-3/100 ft2.
- Consider using a trap crop for thrips, and an indicator plant for TSWV. The trap crop such as fava bean and petunia are attractive to thrips and will alert you to their presence. Petunia cultivars ‘Calypso’, ‘Summer Madness’, and ‘Super Blue Magic’ have been reported to be particularly attractive. Indicator plants may show TSWV symptoms earlier than some other susceptible crops, letting you know there is an outbreak of the virus. Both petunia and gloxinia have been reported to develop lesions 2-3 days after feeding by infected thrips. Adding non-adhesive yellow boards behind petunia (at plant height) may increase their attractiveness to thrips.
- Consider installing exclusion netting over vents and doors of protective structures. Exclusion netting has been shown to delay infestation and reduce the number of thrips in the structure. Ensure the exclusion netting is rated for thrips, which require about a 215 micrometer or smaller opening.
- Remove all plant material and at the end of the season. Then, turn off heating, vent the structures, and allow them to reach freezing for a period. Next, close up the greenhouse, turn on the heat to trick pests into emerging, and then turn the heat off and open them up and allow them to freeze again. Do this three times. In the summer months, you can also close up the greenhouses allow them to “bake” for a week or longer period. Preventing overwintering will be more difficult in year-round greenhouse (especially heated ones) and high-tunnel operations where there is no break in production.
Managing Thrips and/or TSWV
- Rogue all plants that show virus symptoms or are confirmed to have TSWV and remove from the site entirely. Plants should be rogued regardless of if they are in a greenhouse, high tunnel, or the field.
- Apply an insecticide when there is a sudden increase in thrips population on sticky cards and >5 thrips/trap/week are present. Do not wait until numbers are high. Continue insecticide applications at 5-day intervals until numbers decline <5. Pesticides should be carefully rotated to ensure efficacy, as thrips are notorious for developing resistance. However, due to the lifecycle of the thrips, it is best to apply the same class for a 3–4-week period before switching to a new class. Doing so will help reduce the development of resistance. Once a population is under control, continue treating every 7-10 days. Insecticides should be applied in small particles (<100 microns).
- Integrate biological control to help reduce chemical resistance. Biological control is not likely to be sufficient if used alone or when populations are high, but can be used as part of the management program.
Important note: The virus Impatiens Necrotic Spot Virus (INSV) can cause similar symptoms on pepper.
Thank you to Dr. Cheryl Smith and Dr. Anna Wallingford for their expertise.
Sources:
- https://extension.unh.edu/resources/files/Resource002803_Rep4158.pdf
- http://vegetablemdonline.ppath.cornell.edu/factsheets/Virus_SpottedWilt.htm
- https://www.uvm.edu/~entlab/Greenhouse%20IPM/Workshops/2016/Presentations/Eaton-FrankSullivan%20-%20ThripsID-IPMWorkshop2016.pdf
- https://tswv.caes.uga.edu/usda-ramp-project/thrips-vectors.html
- https://images.bugwood.org