Soil pH and Plant Growth
Importance of soil pH
Soil pH is a key factor in determining plant health because it directly affects nutrient availability (Fig 1). If the pH falls outside the optimal range for a given plant, growth can be stunted and yields reduced.
Figure 1: Soil pH, Nutrient Availability, and Plant Health
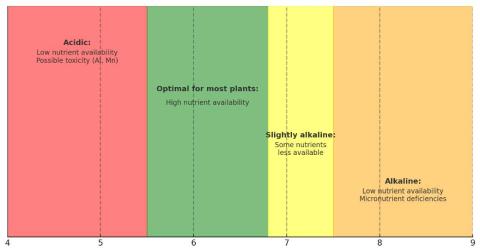
Soil pH
Many NH soils have a pH between 4.5 and 5.5, but most cultivated plants, including grain, fruit, vegetables, flowers and pasture and turf grasses, thrive between 6.0 and 6.8. Some species, such as blueberries and rhododendrons, prefer more acidic (pH 4.5 -5.5) conditions, largely because low pH makes iron more soluble and accessible.
Beneath the soil surface, several chemical, biochemical, and biological reactions transform nutrients, whether naturally present or added through fertilizers and soil amendments, into forms plants can absorb. Soil pH controls many of these reactions by influencing the solubility of nutrients in soil water. The more soluble a nutrient is, the more readily it dissolves into the water around plant roots, allowing for easier uptake and enhancing growth and development.
Biological impacts:
Microbial activity: Soil pH also affects the toxicity of certain elements and the activity of soil microbes. In acidic soils with pH < 5.5, microbial activity slows, reducing the breakdown of organic matter. . Bacteria are generally more prevalent at higher pH, while fungi are more prevalent at lower pH. A pH between 6 and 7 is ideal for maximizing both. This is important because microbes are responsible for making nutrients like nitrogen, phosphorus, and sulfur available to plants.
Pesticide effectiveness: Soil pH can affect how tightly some pesticides bind to clay particles, which in turn influences their effectiveness. It can also impact pesticide breakdown, as some pesticides are degraded by microbes whose activity is affected by pH.
Disease control: Soil pH also helps in combating certain diseases. For example, potato scab is less of a problem at a pH of 5.0 to 5.2, while club root of Brassicas is decreased at a pH of 7.0 to 7.3.
Chemical and Physical impacts:
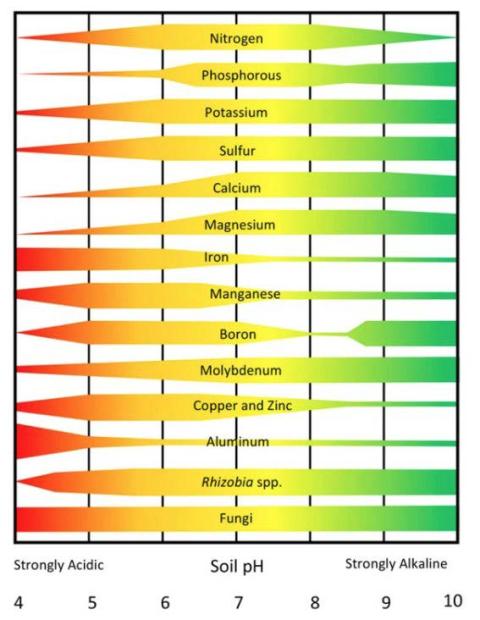
Nutrient availability: Soil pH affects nutrient availability by altering the form of the nutrient and microbial activity in the soil (Figure 2).
When pH drops below 6.0, nutrients like phosphorus, nitrogen, and potassium become less available, and levels of magnesium and calcium often decline. Acidic soils can also release elements such as aluminum, manganese, and iron in harmful amounts toxic to plant roots. Additionally, heavy metals like lead, mercury, copper, and aluminum become more soluble and mobile at lower pH and can potentially leach into groundwater or run off into surface waters.
High pH can be equally problematic. Above 7.5, essential nutrients, including micronutrients like Fe, Cu, Mn, Zn, and B, may again become inaccessible. In garden vegetable beds, very often excessively high pH often results from overuse of lime, wood ashes, or compost. Maintaining the proper pH range ensures nutrients remain available and plants can perform at their best.
Figure 2: The effects of soil pH on the availability of plant nutrients and selected groups of microorganisms
(Picture credit: NDSU)
Adjusting soil pH
A soil test is the best way to determine how much limestone or sulfur to apply. Testing the soil once every 3 years to monitor pH is recommended.
Limestone is the most common material used to raise soil pH. It neutralizes acidity and adds calcium and magnesium. Dolomitic limestone, containing both elements, should be used when magnesium levels are low in soil.
Sulfur is commonly used to lower soil pH. Lowering pH with sulfur is a biological process where soil bacteria convert it to sulfuric acid, and the rate of this conversion depends on temperature, water, and oxygen.
The amount of limestone or sulfur needed to change the pH depends on the soil’s buffering capacity, which is its ability to resist pH changes. Soils with more clay and organic matter have a higher buffering capacity and require more material to change the pH. Sandy soils are easier to change than clay, silt, or organic soils.
Extension Services & Tools That Help NH Farmers Grow
Newsletters: Choose from our many newsletters for production agriculture
Receive Pest Text Alerts - Text UNHIPM to (866) 645-7010



